hu.ufsc.br
T h e n e w e ng l a n d j o u r na l o f m e dic i n e Mandating HPV Vaccination — Private Rights, Public Good To the Editor: Those who oppose mandating lice power” granted to states under the Consti- vaccination against human papillomavirus (HPV) tution’s 10th Amendment permits all jurisdictions as a condition for school entry generally open the to legislate to “protect the p
 at 32 weeks’ gestation, and were oxygen-dependent only untilage 28 days. More recently, underdiagnosed reversible airwaydisease in extremely preterm infants, including those withand without lung disease, has been reporteWhether earlierintervention with bronchodilators improves the long-term re-spiratory outlook for these children remains to be seen, but werecommend that reversible bronchoconstriction be actively as-sessed for and treated, given that such basic treatment is likelyto improve their quality of life. Antenatal maternal smokingand postnatal passive exposurcould possibly play a rolein the observed lung function deficits. Children in the CLDand preterm groups had a greater rate of current parentalsmoking compared with the term-born children, suggestinggreater exposure at least during postnatal life, but we did not
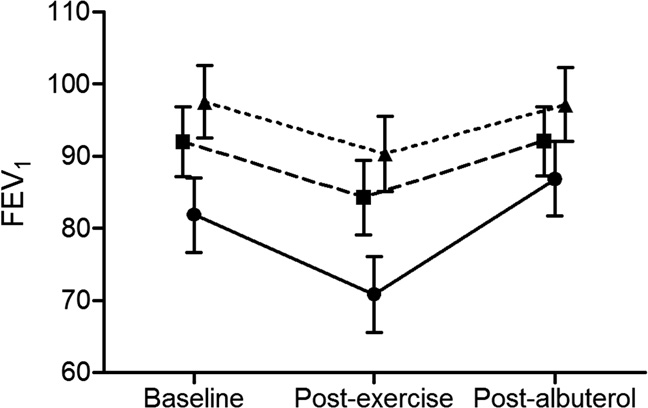
Figure. Mean (95% CI) percentage of predicted FEV
have sufficient data documented during the perinatal period
baseline, after exercise, and after bronchodilator use. Solid
to determine whether children in the CLD group had greater
line, CLD; dashed line, preterm; dotted line, term controls.
at 32 weeks’ gestation, and were oxygen-dependent only untilage 28 days. More recently, underdiagnosed reversible airwaydisease in extremely preterm infants, including those withand without lung disease, has been reporteWhether earlierintervention with bronchodilators improves the long-term re-spiratory outlook for these children remains to be seen, but werecommend that reversible bronchoconstriction be actively as-sessed for and treated, given that such basic treatment is likelyto improve their quality of life. Antenatal maternal smokingand postnatal passive exposurcould possibly play a rolein the observed lung function deficits. Children in the CLDand preterm groups had a greater rate of current parentalsmoking compared with the term-born children, suggestinggreater exposure at least during postnatal life, but we did not
Figure. Mean (95% CI) percentage of predicted FEV
have sufficient data documented during the perinatal period
baseline, after exercise, and after bronchodilator use. Solid
to determine whether children in the CLD group had greater
line, CLD; dashed line, preterm; dotted line, term controls.